Home / VivaDiag™ SARS-CoV-2 Ag Saliva Rapid Test
* Fields required

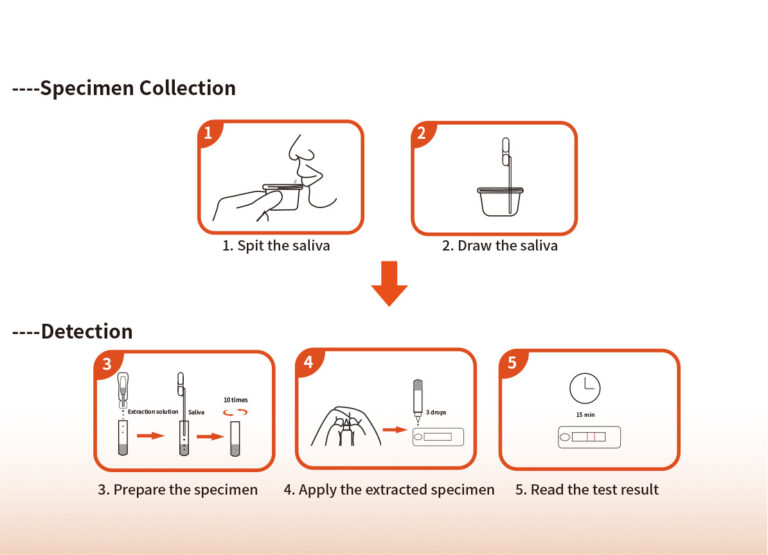

| Test Principle | Immunochromatography |
| Sample Type | Saliva |
| Sample Volume | 60 μL |
| Test Time | 15 min |
| Operation Temperature | 15-30℃ |
| Storage Temperature | 2-30℃ |
| Shelf Life (Unopened) | 24 months |
NOT FOR AT-HOME TESTING
VivaDiag™ SARS-CoV-2 Ag Saliva Rapid Test has ONLY been designed to act as a supplementary test for suspected cases of negative coronavirus nucleic acid detection or in conjunction with nucleic acid detection in the diagnosis of suspected cases. Results from nucleocapsid protein antigen testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 (COVID-19) infection or to inform infection status.
